Dermal Filler Do’s and Don’ts for Wrinkles, Lips and More
6 min readPicture
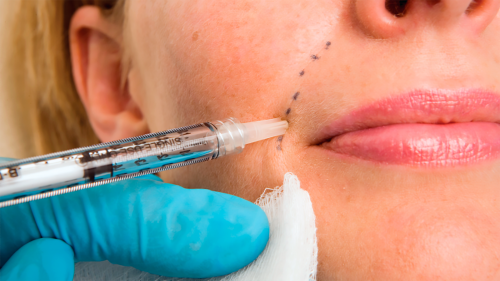
Persons are trying to get remedies to sleek smile lines and crow’s feet and plump up their lips, cheeks, and palms.
Injecting dermal fillers into the confront and palms can boost the visual appeal of facial lines and quantity reduction induced by age or particular health-related ailments. In studies of dermal fillers approved by the U.S. Food and Drug Administration, men and women typically report they are contented with their cure success.
Nonetheless, dermal fillers are not for every person. Dermal fillers may possibly not be suitable for men and women with particular ailments, such as bleeding issues or some allergies. If your health and fitness treatment provider confirms that dermal fillers are an solution for you, know that all health-related goods have advantages and threats. The Fda advises you work with a licensed health and fitness treatment provider who is expert in injecting dermal fillers, experienced about fillers, anatomy, handling difficulties, and most importantly, tells you about the threats and advantages prior to getting cure.
What are dermal fillers?
Dermal fillers are gel-like substances injected beneath the skin. Dermal fillers are intended to generate a smoother or fuller visual appeal, or each.
The Fda regulates dermal fillers as health-related equipment. As noted in scientific trials, the results of most Fda-approved dermal fillers are temporary because they are designed from products that the human body at some point breaks down and absorbs. The injection technique may possibly have to be recurring to retain the preferred impact.
Forms of dermal fillers
Non permanent fillers include the following products:
- Hyaluronic acid, a sugar that is obviously found in the human body
- Calcium hydroxylapatite, a mineral and a important part of bone
- Poly-L-lactic acid (PLLA), a biodegradable, synthetic material
There’s only a single Fda-approved dermal filler that is not absorbed by the human body. It is designed with polymethylmethacrylate (PMMA) beads suspended in a answer that contains bovine (cow) collagen. PMMA beads are small spherical, sleek, plastic beads.
Fda-approved employs of dermal fillers
Dermal fillers are approved for unique employs in men and women aged 22 and older. People employs include:
- Correcting average-to-intense facial wrinkles and skin folds
- Growing fullness of lips, cheeks, chin, beneath-eye hollows, jawline, and back of the hand
- Restoring facial excess fat reduction in men and women with human immunodeficiency virus (HIV)
- Correcting zits scars on the cheek
Fda warnings about unapproved fillers
- The Fda has not approved injectable silicone or any injectable fillers for human body contouring or enhancement. The Fda has warned from getting filler injected into the breasts, buttocks, or areas concerning the muscles. Working with injectable filler for significant-scale human body contouring or human body enhancement can direct to really serious personal injury, such as prolonged-time period soreness, infection, permanent scarring or disfigurement, and even dying.
- The Fda has not approved needle-free of charge equipment for the injection of dermal fillers and warns from working with them to inject hyaluronic acid or other lip and facial fillers. The injectors use large pressure and do not supply sufficient manage more than where filler will be put. Severe accidents and in some scenarios, permanent damage to the skin, lips or eyes have transpired.
- The Fda also warns from shopping for or working with lip or facial fillers that are sold straight to the general public. They are not Fda approved and may possibly be contaminated with substances and infectious organisms. The only Fda-approved dermal fillers are supplied by a prescription for injection by a licensed health and fitness treatment qualified working with a syringe with a needle or a cannula (a small adaptable tubing with a blunt tip that is inserted beneath the skin).
Pitfalls of Fda-approved fillers
As with any health-related technique, there are threats concerned with the use of dermal fillers. Most aspect results noted in scientific trials and post-market place surveillance manifest shortly immediately after injection and go absent inside of a number of weeks. In some scenarios, aspect results may possibly emerge weeks, months, or yrs later on.
Prevalent threats include:
- Bruising
- Redness
- Swelling
- Ache
- Tenderness
- Itching
- Rash
- Issues in carrying out activities (only observed when injected into the back of the hand)
Persons really should be tested for allergies prior to getting dermal fillers designed with particular products, in particular products derived from animals, such as collagen.
Unintended injection into blood vessels
The most really serious danger connected with dermal fillers is accidental injection into a blood vessel. Filler that enters a blood vessel can induce skin necrosis (dying of tissue), stroke, or blindness. Although the odds of this happening are reduced, if it does happen, the ensuing difficulties can be really serious and may possibly be permanent.
Getting rid of Dermal Fillers
If you want to have fillers eliminated or diminished because of aspect results, you may possibly require additional processes to lessen the filler or surgical procedure to take away it. These processes carry their possess threats. Be informed that it may possibly be tough or impossible to take away some filler products.
6 Strategies for Buyers About Injectable Dermal Fillers
- Do work with a licensed health and fitness treatment provider who has practical experience in the fields of dermatology or plastic surgical procedure and is qualified to inject dermal fillers. The provider really should use appropriately labeled, sealed vials or pre-loaded syringes of Fda-approved filler.
- Do request and go through the affected person labeling details on Fda-approved injectable dermal fillers from your licensed health and fitness treatment provider.
- Do know the style of solution to be injected and the achievable threats. Know where just about every solution you will be getting is to be injected. Speak to your licensed health and fitness treatment provider if you have any thoughts.
- Do not purchase dermal fillers that are sold straight to the general public. They may possibly be pretend, contaminated, or not approved for use in the U.S. Fda-approved dermal fillers are indicated for prescription use only.
- Do not inject you with dermal fillers or with needle-free of charge injection “pens.”
- Do not get any style of filler or liquid silicone injected for human body contouring.
Dermal Fillers and Botulinum Toxin Goods
The Fda also has approved botulinum toxin goods such as Botox, Dysport, Xeomin and Jeuveau to take care of facial wrinkles. These goods are not dermal fillers. They are injectable drugs that work by retaining muscles from tightening, so the wrinkles never display as considerably. The risk-free use of dermal fillers in combination with Botox and other remedies has not been evaluated in scientific studies.
Though botulinum toxin goods are derived from the identical bacteria that induce botulism, the amounts made use of for cosmetic applications are purified and lots of orders of magnitude scaled-down.
The Fda has approved these injectable drugs for the temporary advancement in the visual appeal of a single, or most likely many varieties of facial lines, such as frown lines, brow lines, and crow’s feet.
Facet results noted in scientific trials include facial weak point, eyelid drooping, and brow drooping. Other adverse events involved localized soreness, inflammation, reddening, and bruising at the injection web site. In unusual scenarios, injections have resulted in double eyesight, dry eyes, or difficulty swallowing or respiration. The injection of botulinum toxin goods for cosmetic applications is not suggested for use whilst expecting or lactating.
Extra Data
If you have expert a challenge with a dermal filler solution or other solution controlled by the Fda, you can voluntarily report it to MedWatch, the Fda basic safety details and adverse celebration reporting system.






